
Expert Answer
 (1) True.Explanation: An absolute temperature scale is one on which its zero reading coincides with the theoretical absolute zero of temperature i.e.
(1) True.Explanation: An absolute temperature scale is one on which its zero reading coincides with the theoretical absolute zero of temperature i.e.. Thermodynamically this represents the temperature of the minimum energy and beyond this temperature it is not possible for a matter to exist.
K = 273.16 + C=> At,
K = 273.16 - 273.16 = 0Therefore on a scale of Kelvin, the absolute zero temperature would be at 0 K. Hence it is an absolute temperature scale.Rankine is a "Kelvin equivalent" for the Fahrenheit temperature.R = 1.8K=> At 0 K,R = 1.8 x 0 = 0Therefore even in Rankine scale, 0 K represents 0 Rankine, this means that the absolute zero temperature on a Rankine scale will also lies on 0 degree.Hence both of the scales are absolute.In short we can say, Kelvin is an absolute scale related to Celsius and Rankine is an absolute scale related to Fahrenheit.(2) True.Explanation: An isolated system is a system for which the total energy and total mass of the system remains constant, i.e. it does not interact with its surrounding in terms of energy and mass. Hence there is no interaction.(3) (a), (b), (c), (d)Explanation: A control volume is a defined volume in a state which interacts with its surrounding in respect of mass, energy and momentum. In a control volume mass flow inside the system is equals to the mass flow outside the system, hence total mass of the system remains constant. Since, there is a mass interaction with the surrounding, mass can cross the boundary of the control volume with a constant rate. Similarly as mass flow rate, the energy flow rate of the control system is also constant and energy can cross the boundary of the control volume.Since control volume is a fixed region in space, its volume usually does not change with time.(4) (c)Explanation:for all real gases, except monoatomic gases, increases with an increase in the temperature. For monoatomic gases,
remains constant. Monoatomic gases have only 3 degree of freedom unlike polyatomic gases in which degree of freedom varies. Monoatomic gases behaves like an ideal gas with constant value of specific heat.
THERMODYNAMICS PLEASE HELP!!!
Answer the following True or False. (50 pts) Note: DO NOT GUESS!! If you are not sure about it, then circle ‘N’ (N- Not Sure). If you get T or F correctly, you will receive 1.25 point per statement; if you circle ‘N’, because you are not sure, you will get 0.5 point for your honesty. But the maximum number of chosen ‘N’ you can have is 20, you need to think carefully and turn extra ‘N’ into T or F. T F N
1. A closed system always contains the same matter; there is no transfer of matter across its boundary. T F N
2. When a closed system undergoes a process between two specified states, the change in internal energy between these two states is independent of details of the process. T F N
3. Body organs, such as the human heart, whose shapes change as they perform their normal functions CANNOT be studied as control volumes. T F N
4. 1 N equals 1 kg·m/s2 but 1 lbf does not equal 1 lb·ft/s2 . T F N
5. Specific volume, the volume per unit of mass, is an intensive property while volume and mass are extensive properties. T F N
6. If the value of any property of a system changes with time, that system cannot be at steady state. T F N
7. Temperature is the property that is the same for each of two systems when they are in thermal equilibrium. T F N
8. An isolated system is a special type of closed system that does not interact in any way with its surroundings. T F N
9. The net energy transfers by heat are induced only as a result of a temperature difference between a system and its surroundings. T F N
10. The total energy of a open system can change as a result of energy transfer across the system boundary by heat and work and energy transfer accompanying mass flow across the boundary. T F N
11. In principle, expansion work can be evaluated using ? p dV for both actual and quasiequilibrium expansion processes. T F N
12. The change in the internal energy of a system between two states is the change in the total energy of the system between the two states minus the changes of the system’s kinetic and gravitational potential energies between these states. T F N
13. Only changes in the energy of a system between two states have significance; no significance can be attached to the energy at a state. T F N
14. If a system undergoes a process involving heat transfer with its surroundings but no work, that process is said to be adiabatic. T F N
15. For every thermodynamic cycle, the net amount of energy transfer by heat is equal to the net amount of energy transfer by work per cycle. T F N
16. Kinetic and potential energy each are extensive properties of a system. T F N
17. Air always can be regarded as a pure substance. T F N
18. For simple compressible systems, the state principle indicates that the number of independent intensive thermodynamic properties required to fix a state is two. T F N
19. The change in specific volume from saturated liquid to saturated vapor, (vg – vf), at a specified saturation pressure increases as the pressure decreases. T F N
20. A two-phase liquid-vapor mixture with equal volumes of saturated liquid and saturated vapor has a quality of 50%. T F N
21. The following assumptions apply for a liquid modeled as incompressible: The specific volume (density) is constant and the specific enthalpy is a function only of temperature. T F N
22. The maximum thermal efficiency of any power cycle operating between hot and cold thermal reservoirs at 1000 oC and 500 oC, respectively, is 50%. T F N
23. A process of a closed system that violates the second law of thermodynamics necessarily violates that first law of thermodynamics. T F N
24. One statement of the second law of thermodynamics recognizes that the extensive property entropy is produced within systems whenever friction and other nonidealties are present there. T F N
25. In principle, the Clausius inequality is applicable to any thermodynamic cycle. T F N
26. When a net amount of work is done on a system undergoing an internally reversible process, a net heat transfer from the system necessarily occurs. T F N
27. A product website claims that a heat pump capable of maintaining a dwelling at 21 oC on a day when the outside temperature is 0 oC has a coefficient of performance of 3.5. Still, such a claim is not in accord the second law of thermodynamic. T F N
28. The second Carnot corollary states that all power cycles operating between the same two thermal reservoirs have the same thermal efficiency regardless of internally reversible or irreversible processes. T F N
29. The energy of an isolated system remains constant, but its entropy can only decrease. T F N
30. Internally reversible processes do not actually occur but serve as hypothetical limiting cases as internal irreversibilities are reduced further and further. T F N
31. For closed systems undergoing processes involving internal irreversibilities, both entropy change and entropy production are positive in value. T F N
32. Entropy change of a closed system during a process can be greater than, equal to, or less than zero. T F N
33. For specified inlet state, exit pressure, and mass flow rate, the power input required by a compressor operating at steady state is less than that if compression occurred isentropically. T F N
34. The steady-state form of the control volume entropy balance requires that the total rate at which entropy is transferred out of control volume be less than the total rate at which entropy enters. T F N
35. For a gas modeled as an ideal gas, the specific internal energy, enthalpy, and entropy all depend on temperature only. T F N
36. The increase of entropy principle states that the only processes of an isolated system are those for which its entropy increases. T F N
37. The only entropy transfer to, or from, control volumes is that accompanying heat transfer. T F N
38. For a specified inlet state, exit pressure, and mass flow rate, the power developed by a turbine operating at steady state is less than if expansion occurred isentropically. T F N
39. The entropy change between states of air modeled as an ideal gas can be directly read from Table A-22 only when pressure at these states is the same. T F N
40. When a system undergoes a Carnot cycle, entropy is produced within the system
Expert Answer
General guidance
Concepts and reason
Closed system:
A closed system does not exchange the mass between the system and the surrounding. However, it transfer of energy between the system and the surrounding does take place in case of closed system.
A change in internal energy is state function not a path function. Therefore, it depends only on the initial and final state of the system. The path followed by the thermodynamic system for any thermodynamic process does not affect the change in internal energy.
Control volume refers to the volume in space, which is under consideration for the thermodynamic analysis.
A equivalent magnitude of gravitational acceleration in 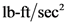 is equal to
is equal to 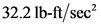 .
.
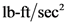 is equal to
is equal to 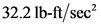 .
.
Fundamentals
Some amount of matter or a region in space upon which an analysis is done is called the thermodynamic system. It mainly of three types:
Closed system: A closed system is the one that does not transfer the mass across the boundary. It does transfer some energy across the boundary.
Open system: Energy transfer and mass transfer both takes place in open type of thermodynamic system.
Isolated system: Neither mass transfer nor energy transfer takes place in isolated thermodynamic system.
A point function only depends on the initial and final state of the thermodynamic system. While a path function also consider the path followed by the system during any thermodynamic process.
A thermodynamic analysis is done on particular region in space. The volume of this specific region is called as the control volume of the thermodynamic system.
Show less
FIRST STEP | ALL STEPS | ANSWER ONLY
Step-by-step
Step 1 of 4
(1)
For any closed type of thermodynamic system, there is no mass exchange takes place between the system and surrounding for any thermodynamic system. Therefore, the amount of matter remains constant throughout the thermodynamic process. However, the energy transfer does take across the boundary.
Therefore, the statement, closed system has no matter transfer is true.
Part 1
The given statement is True (T).
Explanation | Common mistakes | Hint for next step
In case of closed thermodynamic system, the physical exchange of matter between the system and the surrounding does not takes place as the system is completely closed.
Step 2 of 4
(2)
The detailing about the process is not required for the change in the internal energy of the system as the internal energy is a point function. It depends only on the initial and final state of the thermodynamic system. The path being followed does not affect the magnitude of the change in the internal energy of the system.
Therefore, the change in internal energy between these two states is independent of the detail of the process.
Part 2
The given statement is True (T).
Explanation | Common mistakes | Hint for next step
The internal energy is a state function. It depends only on the initial and final state of the thermodynamic process. Therefore, the details about the process are not required for the closed system.
Step 3 of 4
(3)
Control volume refers to the volume in space, which is under consideration for the thermodynamic analysis. Any physical object, which has certain volume, can be taken under consideration for thermodynamic analysis. Therefore, body organs can be studied as the control volume despite of the change in its shape.
Therefore, the statement, body functions cannot be studied as control volumes is false.
Part 3
The given statement is False (F).
Explanation | Common mistakes | Hint for next step
The change in the volume of the body organs due to the shape change does not disqualify it from considering it as control volume. It can be studied as the control volume and the thermodynamic analysis can be done on it.
Step 4 of 4
(4)
A magnitude of gravitational acceleration in terms of 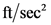 is
is 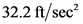 .
.
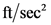 is
is 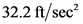 .
.
Therefore, equivalent value of 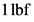 in
in 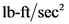 is given as:
is given as:
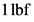 in
in 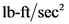 is given as:
is given as: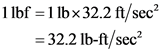
Therefore, the force required to accelerate the mass of 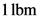 to an acceleration of
to an acceleration of 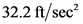 is
is 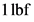 .
.
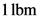 to an acceleration of
to an acceleration of 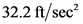 is
is 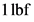 .
.
Therefore, the statement, 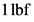 is not equal to
is not equal to 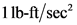 is true.
is true.
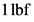 is not equal to
is not equal to 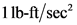 is true.
is true.
Part 4
The given statement is True (T).
Explanation | Common mistakes
The correct option is T as 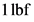 is equal to
is equal to 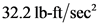 . Therefore, the given statement is true.
. Therefore, the given statement is true.
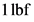 is equal to
is equal to 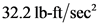 . Therefore, the given statement is true.
. Therefore, the given statement is true.Answer
Part 1
The given statement is True (T).
Part 2
The given statement is True (T).
Part 3
The given statement is False (F).
Part 4
The given statement is True (T).

ليست هناك تعليقات:
إرسال تعليق