Problem
Estimate the flash point of a solution of 50 mol % water and 50 mol % ethanol.
Step-by-step solution
Step 1 of 4
The flash point of a liquid is the temperature at which the liquid has sufficient vapour pressure to just form a flammable atmosphere in equilibrium with the liquid. Hence, the temperature at which the fluid ignites is called as the flash point.
Comment Step 2 of 4
By using Antoine equation we can calculate the saturation vapor pressure
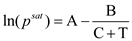
From Appendix B, the flash point temperature for ethanol is
 .
.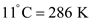
Hence,
At the flash point temperature the saturation vapour pressure is,

Comment Step 3 of 4
By using Raoult’s law we can calculate the partial pressure,

Thus, the partial pressure is
 .
.Comment Step 4 of 4
The temperature corresponding to 57.1 mm Hg can be calculated by using Antoine’s Equation.
From Appendix E for Ethanol,

Hence, the temperature corresponding to 57.1 mm Hg is
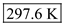 .
.Therefore, 297.6 K corresponds to
 that is
that is .
.Thus, the flash point of ethanol solution is
 .
.Comment
