Sodium chloride is added to pure water to increase its ionic strength from es-sentially zero to 0.1 mol/L. The solution remains at 250 and pH 7.0 throughout the process.
A) How many moles of water molecules dissociate per liter of solution when the salt is added?
B) By what fraction does the concentration of H+ increase?
C) By what fraction does the activity of H+ increase?
Expert Answer
 Comment Explain why we always assume that activity coefficients are 1.0 for pure solids, regardless of the ionic strength of the solution in which they are found
Comment Explain why we always assume that activity coefficients are 1.0 for pure solids, regardless of the ionic strength of the solution in which they are found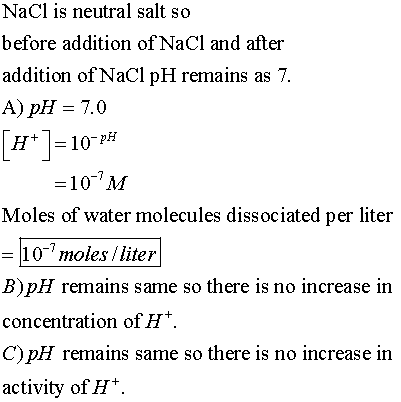
Expert Answer

For solids or liquids, as mass decreases the volume also decreases by a proportional amount.
So, example:
1 kg of water and 0.1 mL water both have molar densities of 55.5 mol/L.
So molar density variations are minimum and hence the variations in molar activity is minimum.So the activity coefficient is taken as unity, since we do not need to consider this everytime we analyse the system!



ليست هناك تعليقات:
إرسال تعليق